Abstract

Objective: PDGFRB fusions in acute lymphoblastic leukemia (ALL) is rare. PDGFRB fusions was mainly observed in B-ALL, but also in T-ALL. Previous studies were mostly limited to anecdotal cases, suggesting that this subtype of ALL had low rate of remission and poor prognosis. The addition of tyrosine kinase inhibitor (TKI) to intensive chemotherapy may improve the outcome, but lack of long-term survival data. Herein, we retrospectively analyzed the clinical characteristics, survival and prognostic factors for 28 pediatric patients with PDGFRB fusions ALL.
Methods: This multicenter, retrospective study included pediatric patients with newly diagnosed PDGFRB fusion ALL after treatments according to the CCCG-ALL-2015 and CCCG-ALL-2020 protocols from April 2015 to April 2022 in 10 hospitals in China. All patients completed the followed up. The baseline characteristics and treatment outcomes of these patients, including complete remission, drug resistance, relapse, treatment-related mortality and minimal residual disease (MRD) at end of induction therapy, as well as hematopoietic stem cell transplantation (HSCT) were collected. The outcome was compared between patients treated with and without TKI. Fisher test and nonparametric rank sum test were used for comparative analysis. 4-year event-free survival (EFS) and 4-year overall survival (OS) were estimated by use of Kaplan-Meier methods, and the 4-year cumulative incidence of relapse was calculated by use of a competing risk model gray test. Univariate and multivariate analyses were performed by use of Cox regression.
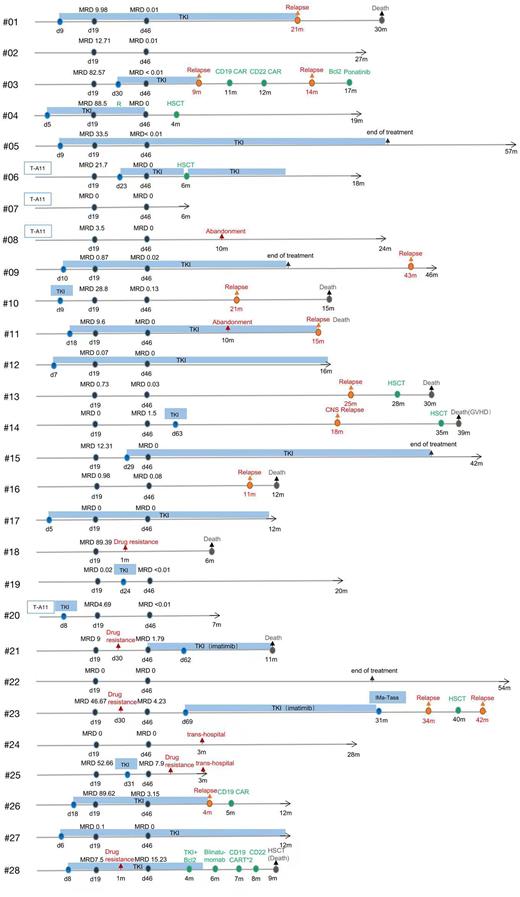
Results: We identified 28 pediatric patients with newly diagnosed PDGFRB fusion ALL, which accounted for approximately 0.5% of childhood ALL. In all 28 patients, 21 (75%) were male and 7 (25%) were female; the median age was 10 (1-16) years old; median white blood cell (WBC) was 29.8 (1.6-627.7) × 10^9/L; 24 (85.7%) were acute B-lymphoblastic leukemia and 4 (14.3%) were acute T-lymphoblastic leukemia; one case (3.5%) was observed accompanied by the CNS involvement at diagnosis. Twenty of the patients received TKI treatment. Only 6 patients underwent hematopoietic stem cell transplantation. For all 28 patients, the median follow-up was 25.5 (3-77) months, the 4-year EFS was 29.2% (95% CI, 13.6-36.4), the 4-year OS was 42.1% (95% CI, 30.6-61.4), and the 4-year cumulative incidence of relapse was 58% (95% CI, 45·6-70·1). Patients in TKI group had a high rate of complete remission after induction therapy compared with those in no-TKI group (90% vs 63.6%). However, no significant differences were observed in the 4-year EFS and 4-year OS between TKI group and no-TKI group (EFS, 21.1% vs 43.8%, P=0.925; OS, 50.1% vs 36.5%, P=0.807). The cumulative incidences of relapse between these two groups also have no remarkable differences (P >0.05). Among the 6 patients who underwent HSCT, 2 patients who achieved CR1 survived and 4 died. Univariate analysis showed that minimal residual disease (MRD) ≥10-4 after induction therapy for D46 and IKZF1 positive had a poor prognosis (all P <0.05). MRD≥10-4 at the end of induction therapy is an independent risk factor for poor prognosis (hazard ratio of event-free, 9.101 [95% CI 2.03-40.86], P =0·004, compared to those with a MRD of <10-4).
Conclusion: Pediatric ALL patients with PDGFRB fusions have a poor outcome. This subtype of ALL is associated with high-risk clinical features such as older age, high WBC count at diagnosis, high MRD after induction therapy, and increased risk of leukemia relapse. The addition of TKI to chemotherapy can improve the early remission rate of treatment, but does not improve the long-term survival. Profitably, the TKI treatment can provide an opportunity for subsequent treatment, including HSCT. Additionally, HSCT has indeed potential to reduce the relapse rate for this subtype patients. HSCT is recommended for this subtype patients who achieve CR1. The MRD≥10-4 or more at the end of induction therapy was independently predictive factor for unfavorable outcome and stratified treatment based on MRD may improve survival of these patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal